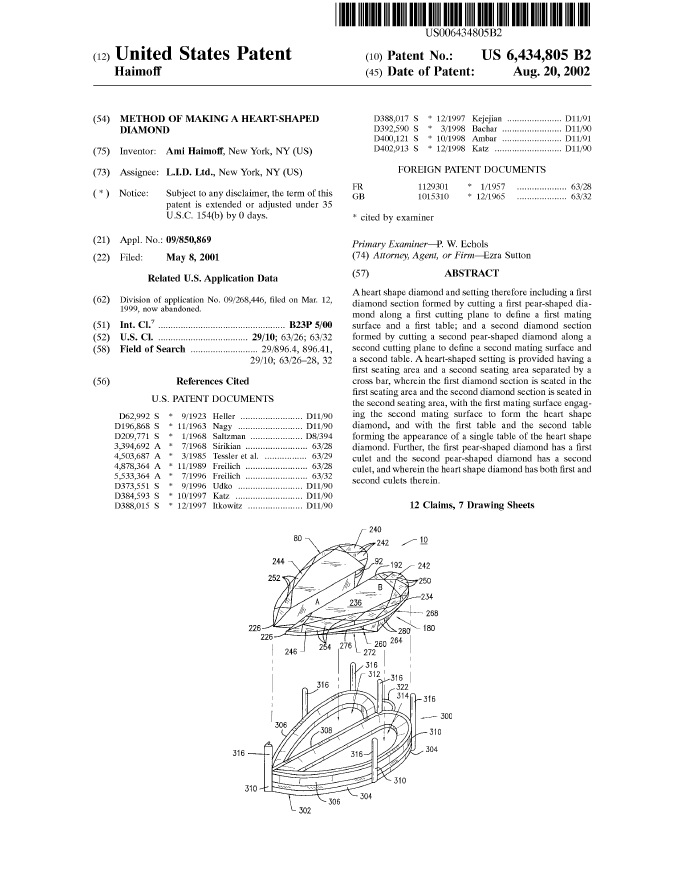
In Rembrandt Wireless Technologies, LP v. Samsung Electronics CO., LTD., [2016-1729] (April 17, 2017), the Federal Circuit affirmed the district court’s claim construction and denial of JMOL, but vacated $15.7 million in damages and remanded because of a lack of patent marking.
In affirming the claim construction, the Federal Circuit found unambiguous statements in the prosecution history defined the meaning of “different types” of modulation, and Samsung’s arguments to the contrary do not diminish the unambiguous statements in
the prosecution history. Samsung also attacked the use of the term “i.e.,” which Samsung argued introduces an exemplary item in a set, but the Federal Circuit found that the use of i.e. was “i.e., is often definitional, noting that i.e. is Latin for id est, which means “that is.” The Federal Circuit also rejected Samsung’s reference to the broader construction in a related IPR, noting that the construction is not binding, and that IPR proceedings operates under a broader claim construction standard than the federal
courts.
In affirming the non-obviousness determination, the Federal Circuit rejected Samsung’s argument that it was a claim construction issue of the scope of “different types,” and characterizing it as a factual question of whether the reference disclosed “different types” of modulation. The Federal Circuit found that there was substantial evidence to support the jury’s presumed finding underpinning the non-obviousness determination.
On the marking issue prior to trial, Samsung moved to limit Rembrandt’s potential
damages award based on its failure to mark products covered by previously-asserted claim 40 of the ’580 patent. Specifically, Rembrandt had licensed the ’580 patent to Zhone Technologies, Inc., and the license agreement between Rembrandt and Zhone did not require Zhone to mark its products with the patent number. Pursuant to the patent marking statute, 35 U.S.C. § 287, Samsung sought to limit Rembrandt’s damages to those incurred after Samsung received notice of Rembrandt’s patents, which, according to Samsung, occurred when Rembrandt filed its complaint. Eight days later, Rembrandt withdrew claim 40 from its infringement allegations and filed a statutory disclaimer pursuant to 35 U.S.C. § 253(a) and 37 C.F.R. § 1.321(a), disclaiming claim 40 in the U.S. Patent and Trademark Office. The court accepted
Rembrandt’s argument that any prior obligation to mark products embodying claim 40 vanished once it disclaimed claim 40.
The Federal Circuit held that Rembrandt cannot use disclaimer to avoid the marking requirement in 35 U.S.C. § 287, and vacated the judgment of the district court as it relates to marking. While marking under 35 U.S.C. § 287 is permissive, there is a consequence if the patent owner chooses not to mark:
In the event of failure so to mark, no damages shall be recovered by the patentee in any action for infringement, except on proof that the infringer was notified of the infringement and continued to infringe thereafter, in which event damages may be recovered only for infringement occurring after such notice.
The Federal Circuit said that the marking statute protects the public’s ability to exploit an unmarked product’s features without liability for damages until a patentee provides
either constructive notice through marking or actual notice. The Federal Circuit added that allowing Rembrandt to use disclaimer to avoid the consequence of its failure to mark undermines the marking statute’s public notice function. While a disclaimer can disclaim the rights of the patent owner, the Federal Circuit said it has never held that a patent owner’s disclaimer relinquishes the rights of the public. Considering the rights held by the public, the Federal Circuit held that disclaimer cannot serve to retroactively dissolve the § 287(a) marking requirement for a patentee to collect pre-notice damages.
Before the district court, Rembrandt argued, consistent with its disclaimer position, that the marking requirement is determined on a claim by claim basis, rather than on a patent-by-patent basis. The Federal Circuit left this question for the district to determine in the first instance on remand.