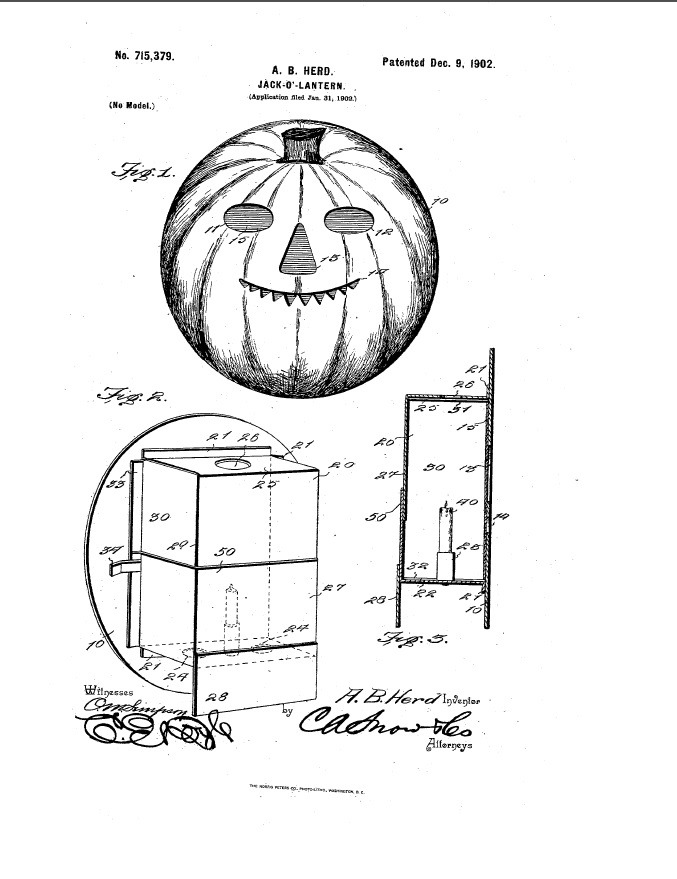
On December 9, 1902 U.S. Patent No. 7157339 issued to A. B. Herd on a Jack-O’-Lantern.
Category Archives: Uncategorized
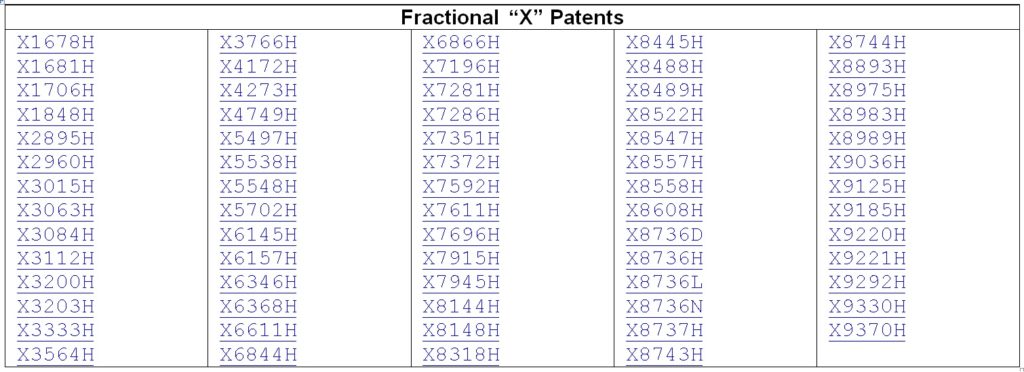
Fractional Patents
No, a fractional patent is not what you have left over after the PTAB gets a hold of your patent. Fractional patents are actually patents with fractional numbers. The practice started back when the USPTO was trying to reassemble the collection of patents issued before the Office numbered patents. These pre-number era patents are known as the “X” patents, and as the Office was assigning these patents numbers, they would occasionally come across patents whose issue dates were between already numbered patents. In order to keep the chronological sequence, the USPTO needed to issue fractional numbers.
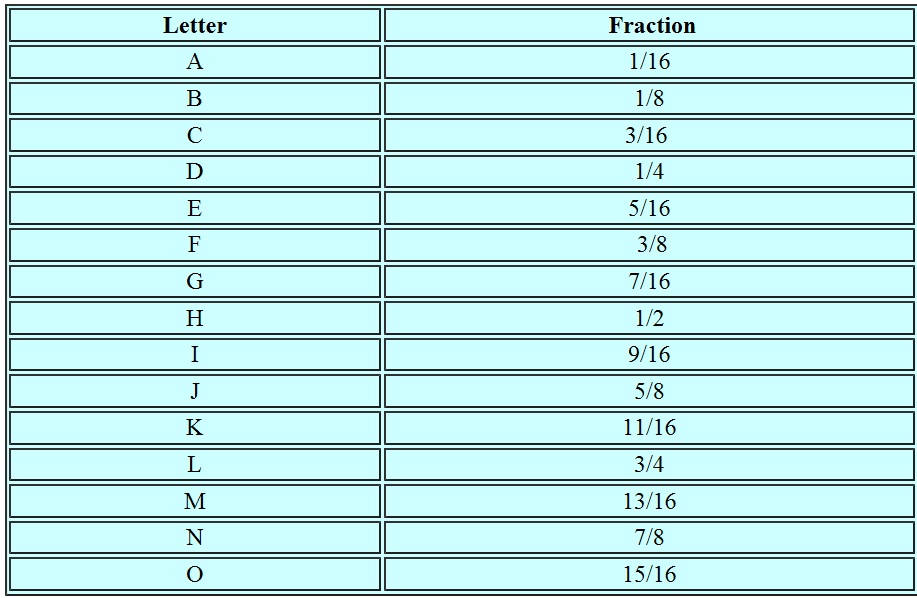
In fact the USPTO even created a letter code system to identify the fractions:
The surprising thing is that the fractional number scheme continued from time to time even after the USPTO began numbering patents. 126½, 1400½, RE1217½, RE1242½, D1093½, 2712152½, 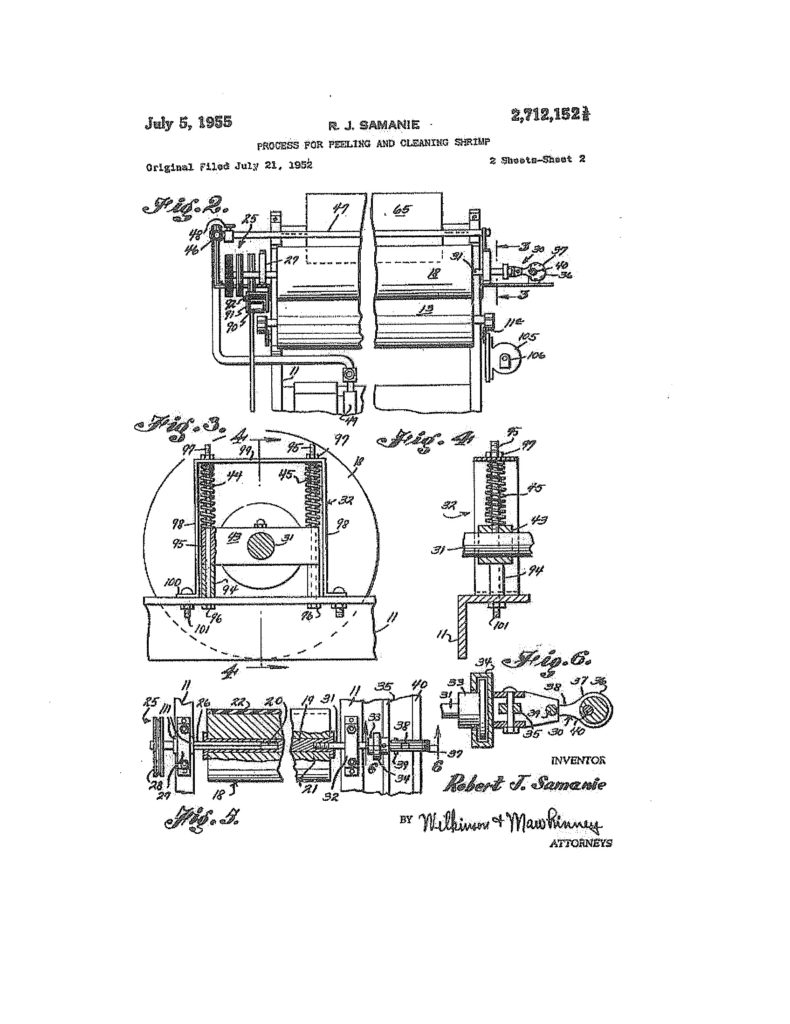 3262124½,
3262124½, 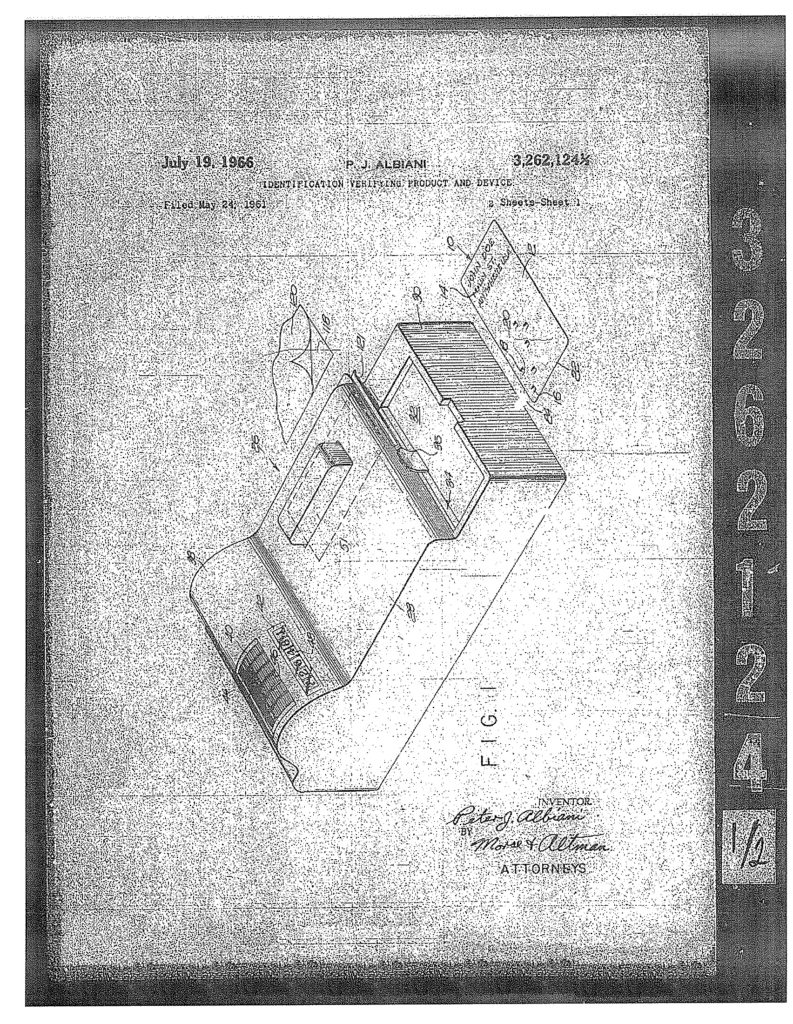
and D90793½.
Federal Circuit Vacates Another IPR as Time-Barred
Luminara Worldwide, LLC v. Iancu, [2017-1629, 2017-1631, 2017-1633] (August 16, 2018), the Federal Circuit affirmed the PTAB’s invalidation of claims of two of Luminara’s patent, and as to the third, it vacated the Final Written Decision, and remanding for dismissal of that IPR, because of the section §315(b) time-bar.
In instituting the IPR, the Board rejected the argument was untimely under §315(b) because the first action had been voluntarily dismissed without prejudice. Because the section 315(b) time-bar applies when the underlying complaint alleging infringement has
been voluntarily dismissed without prejudice, the Federal Circuit said the Board erred in instituting the IPR challenging the ’319 patent, vacating the Board’s final written decision as to the ’319 IPR and remand for dismissal of that IPR.
As to the obviousness in the other two IPRs, the Federal Circuit found that these were supported by substantial evidence.
There Must be at least Some “Causal Connection” Between the Misconduct and the Fee Award
In In re Rembrandt Technologies LP Patent Litigation, [2017-1784] (August 15, 2018), the Federal Circuit held that the district court did not abuse its discretion in deeming this case exceptional, but that the court erred by failing to analyze fully the connection between the fees awarded and Rembrandt’s misconduct, and vacated the district court’s fee award, and remanded for further proceedings.
Rembrandt asserted two patents that were revived improperly; allowed spoliation of evidence; improperly gave consultants an interest contingent on the litigation outcome; and threatened AOPs with a baseless injunction demand. The district court determined that the case was “indeed exceptional” for three reasons. First, the court found that “the evidence shows that Rembrandt improperly compensated its fact witnesses, in violation of ethical rules of conduct.” Second, the court was “convinced that Rembrandt engaged in (or failed to prevent) widespread document spoliation over a number of years.” Finally, the court found that “Rembrandt should have known that the ‘revived patents’ were unenforceable.” The district court ultimately ordered Rembrandt to pay more than $51 million in fees to all Appellees.
On the exceptionality finding, The Federal Circuit said that Rembrandt raised strong arguments with respect to the district court’s factual findings. The Federal Circuit said that the district court’s remarkably terse orders (four pages) shed little light on its justifications for its decisions on these fact-intensive issues. But abuse of discretion is a deferential standard. On the record before it, the Federal Circuit could not say that any of the district court’s findings was based “on an erroneous view of the law or on a clearly erroneous assessment of the evidence.”
On the issue of the amount of the award, Rembrandt raised no specific objections to Appellees’ tabulations of the hours they expended; nor did Rembrandt contend that
Appellees should have calculated fees using a lower hourly rate. Rembrandt instead argued that the fee award is excessive and unreasonable because the district
court failed to establish a causal connection between the claimed misconduct and the fees awarded, and the Federal Circuit agreed. The district court did not explain why
an award of almost all fees was warranted or whether it had accepted appellee’s argument that the pervasive misconduct justified an award of all fees. The district court, by and large, did not even attempt to assess which issues the claimed misconduct affected.
Even if Rembrandt’s misconduct, taken as a whole, rendered the case exceptional, the district court was required to establish at least some “causal connection” between the misconduct and the fee award. What the district court did —award all fees with
no explanation whatsoever of such a causal connection—was not enough. The Federal Circuit thus vacated the district court’s fee award and remanded for the district court to conduct the appropriate analysis in the first instance.
Distribution of Catalogs at a Tradeshow Was Accessible with Reasonable Diligence, and was a Printed Publication under 102(b)
In GoPro, Inc. v. Contour IP Holding LLC, [2017-1894, 2017-1936] (July 27, 2018), the Federal Circuit vacated and remanded the PTAB’s decisions in IPR2015-01078 and IPR2015-01080 that U.S. Patent Nos. 8,890,954 and 8,896,694 relating to action sport video cameras or camcorders that are configured for remote image acquisition control and viewing.
The focus of the appeal was whether a Go Pro catalog, which disclosed a digital camera linked to a wireless viewfinder/controller that allows for a user preview before recording, was prior art. While the PTAB considered the catalog to be prior art in its decision instituting the IPRs, Contour argued that the catalog was not a printed publication, and the PTAB agreed, finding that that the GoPro had not shown that its Catalog was disseminated or otherwise made available to the extent that persons interested and ordinarily skilled in the subject matter or art and exercising reasonable diligence could have located it.
The Board found all the evidence presented by GoPro credible, but explained that GoPro did not provide evidence that the dealer show was advertised or announced to the public, such that a person interested and ordinarily skilled in the art from the public would have known about
it. The Board found that a person of ordinary skill in the art would not be interested in the show where the catalogs were distributed because it was not an academic conference or camera industry conference, but
rather a dealer show for action sports vehicles like motorcycles, motorbikes, ATVs, snowmobiles, and watercraft.
The Federal Circuit disagreed, saying that the case law regarding accessibility is not as narrow as the Board interpreted it. The Board focused on only one of several factors that are relevant to determining
public accessibility in the context of materials distributed at conferences or meetings, but cited no case where the Federal Circuit held that the the expertise of the target audience was dispositive.
The Federal Circuit said that the fact that the dealer show was focused on action sports vehicles was not preclusive of persons ordinarily skilled in the art from attending to see what POV digital cameras were being advertised and displayed. The Federal Circuit noted that a primary purpose of POV cameras is for use on vehicles in extreme action environments, such as the ones advertised at the show. The Federal Circuit further noted that the The vendor list provided by Go Pro listed a number of vendors who likely sell, produce and/or have a professional interest in digital video cameras, and that the show was directed to action sports vehicles and accessories (emphasis in original).
The Federal Circuit said that the standard for public accessibility is one of “reasonable diligence” to locate the information by “interested members of the relevant public.” A dealer show focused on extreme sports vehicles is an obvious forum for POV action sports cameras. The Federal Circuit concluded that GoPro met its burden to show that its catalog is a printed publication under §102(b). The Federal Circuit vacated vacate the Board’s decision that claims 1–20 of the
’694 patent and claims 1, 2, and 11–30 of the ’954 patent were not unpatentable and remanded for further proceedings consistent with its opinion.
Materials Available at Internet Identified in Federal Register Before the Critical Date Were Printed Publications
In Jazz Pharmaceuticals, Inc. v. Amneal Pharmaceuticals, LLC, [2017-1671, 2017-1673, 2017-1674, 2017-1675, 2017-1676, 2017-1677, 2017-2075] (July 13, 2018), affirmed the PTAB decision that claims of U.S. Patent Nos. 7,668,730, 7,765,106, 7,765,107, 7,895,059, 8,589,182, 8,457,988, and 8,731,963 on a drug distribution system for
tracking prescriptions of a “sensitive drug” were invalid for obviousness.
The Board’s obviousness determination relied upon certain materials from the regulatory review process, and primary the issue on appeal is whether these materials were sufficiently accessible to the public to
constitute prior art. The Board determined that the materials were publicly accessible on an FDA website over two months prior to the critical date. The Board found that a person of ordinary skill in the art that a person of ordinary skill “would have been familiar with
the Federal Register and motivated to look for notices related to drug distribution, safety, or abuse prevention,” and that a skilled artisan would have known that Xyrem® contained a “sensitive drug,” providing a person of ordinary skill with “sufficient motivation to have located the Federal Register Notice and FDA web-site. Such a person would have been capable of finding the Notice and following the links to the materials in the Notice.
The Federal Circuit said that a reference is considered publicly accessible upon a satisfactory showing that such document has been disseminated or otherwise made available to the extent that persons
interested and ordinarily skilled in the subject matter or art, exercising reasonable diligence, can locate it. If accessibility is proved, there is no
requirement to show that particular members of the public actually received the information.
The Federal Circuit agreed with Amneal that substantial evidence supports the Board’s finding that the materials were publicly accessible. The Federal Circuit examined the public notices, and observed that this is not the first time it has considered whether
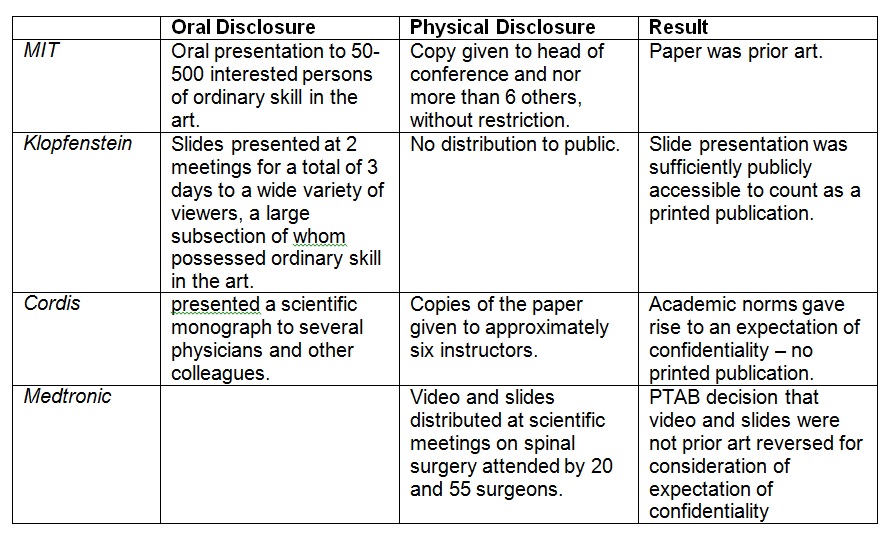
materials disclosed in association with meetings or conferences
were “printed publications,” citing MIT, Klopfenstein, Cordis, and Medtronic.
Comparing the facts of the present case to those in MIT,
Klopfenstein, and Medtronic confirmed that the materials were disseminated more broadly and for a longer duration to persons of ordinary skill than the materials disclosed at individual meetings in those cases. In addition, unlike in Cordis, disclosure through public domain sources such as the Federal Register and a public federal
agency website plainly indicates that there was no reasonable
expectation that the materials would remain confidential.
The Federal Circuit found that:
- The breadth of the dissemination here to persons of ordinary skill was significant
- The materials were available online for a substantial time before the critical date
- The materials were distributed via public domain sources with no possible expectation that the materials would remain confidential or not be copied
The Federal Circuit said that indexing or searchability is unnecessary for a reference to be a printed publication under § 102(b), although it noted that the Federal Register was meaningfully indexed. However the Federal Circuit cautioned that it was not applying per se rule that every notice in the Federal Register satisfies the requirements for prior art, nor was it endorsing a rule that would supplant the case-by-case
inquiry consistently applied throughout our case law. The Federal Circuit did reiterate that if accessibility is proved, there is no requirement to show that particular members of the public actually received the information.
The Federal Circuit went on to affirm the Board’s claim construction, and ultimate conclusion of obviousness
.
Federal Circuit Makes Minor Correction to May 1 Opinion in Texas Advanced Optoelectronic Solutions
In Texas Advanced Optoelectronic Solutions, Inc. v. Renesas Electronics America, Inc., [2016-2121, 2016-2208, 2016-2235] (July 9, 2018), the Federal Circuit, after a petition for rehearing, reissued its opinion affirming liability for trade secret misappropriation and patent infringement, but vacating the monetary awards, and remanding for further proceedings. The reissued opinion only differs from the early opinion in the fifth paragraph of Section III.A.
Third Time’s The Charm: Federal Circuit Remands for Third Damages Trial Because Patent Owner Did Not Prove Use of Entire Market Value was Appropriate
In Power Integrations, Inc. v. Fairchild Semiconductor International, Inc., [2016-2691, 2017-1875] (July 3, 2018), the Federal Circuit affirm the district court’s judgments of infringement of U.S. Patent Nos. 6,212,079 and 6,538,908, but concluded that the entire market value rule cannot be used here to calculate damages and vacated the
damages award and remanded for further proceedings.
The patents in suit related to power supply controller chips used in power
supplies, such as chargers for electronic devices. While the case was pending in the district court, the Federal Circuit in VirnetX, Inc. v. Cisco Systems, Inc.,explained that simply identifying the smallest salable unit is not necessarily sufficient to satisfy a patentee’s obligation to apportion for multi-component products with significant unpatented features. Because Power Integrations’ royalty calculation in the first trial did not apportion beyond the “smallest salable unit” and Power Integrations had disclaimed reliance on the entire market value rule, the district court granted a new trial on the
issue of damages. The district court excluded Power Integrations’ expert testimony based on apportionment, but allowed its expert to present testimony based on the entire market value rule. The jury awarded $139.8 million in damages, based on damages
testimony that relied solely on the entire market value rule The district court denied Fairchild’s motion for judgment as a matter of law, or in the alternative for a new trial.
The Federal Circuit affirmed infringement of U.S. Patent No. 6,212,079 finding the verdict supported by substantial evidence, noting that the jury could have properly concluded that the claim terms “fixed frequency” and “non-varying” left open the
possibility for minor frequency variations due to operating conditions.
The Federal Circuit also affirmed infringement of U.S. Patent No. 6,538,908 under the doctrine of equivalents, agreeing that adjusting a voltage limit of a power switch was an infringing equivalent of adjusting a current limit of a power switch. The Federal noted that Circuit Power Integrations’ expert testified that a value of voltage qualifies as a “value of current” because under Ohm’s Law, current is equal to voltage divided by
resistance. The Federal Circuit also affirmed that equivalence was not precluded by prosecution history, and in particular to arguments distinguishing current and voltage in another application sharing the same specification. The Federal Circuit said that the claim language on its face is different than the language of the claims to which the prosecution argument was directed, and concluded that Fairchild failed to establish that the prosecution history is sufficiently clear as to create an estoppel.
On the issue of damages, the Federal Circuit agreed with Fairchild that the district court should have granted the new trial motion. The Federal Circuit said that a patentee is only entitled to a reasonable royalty attributable to the infringing features, and must
in every case give evidence tending to separate or apportion the defendant’s profits and the patentee’s damages between the patented feature and the unpatented features. In the context of a utility patent, it is only the patented technology that is taken from the owner, so the value to be determined is only the value that the infringing features contribute to the value of an accused product.
Where multicomponent products are accused of infringement, the royalty base should not be larger than the smallest salable unit embodying the patented invention. Use of the entire market value of a multi-component product that includes a patented
component cannot help but skew the damages horizon for the jury, regardless of the contribution of the patented component to this revenue. The Federal Circuit said that Admission of evidence of the entire market value only serves to make a patentee’s proffered damages amount appear modest by comparison, and to artificially inflate the jury’s damages calculation beyond that which is adequate to compensate for the infringement.
The Federal Circuit noted that the entire market value rule allows for the recovery of damages based on the value of an entire apparatus containing several features, when the feature patented constitutes the basis for consumer demand. However if the product has other valuable features that also contribute to driving consumer demand—patented or unpatented—then the damages for patent infringement must be apportioned to reflect only the value of the patented feature.
In the present case, both parties agreed that the accused products contained other valuable features as well, yet Power Integrations presented no evidence about the
effect of those features on consumer demand or the extent to which those features were responsible for the products’ value.
The Federal Circuit said that the entire market value rule is appropriate only when the
patented feature is the sole driver of customer demand or substantially creates the value of the component parts. It is not enough to merely show that the patented feature is viewed as essential, that a product would not be commercially viable without the patented feature, or that consumers would not purchase the product without the
patented feature. The Federal Circuit said that when the product contains
other valuable features, the patentee must prove that those other features did not influence purchasing decisions.
Because the evidence presented by Power Integrations was insufficient as a matter of law to invoke the entire market value rule, the Federal Circuit vacated the award of damages and remand for a new trial.
Board’s Decision Did not Change Theories Simply Because it Used Different Language than the Petition
In Sirona Dental Systems GmbH v. Institut Straumann AG, [2017-1341, 2017-1403](June 19, 2018), the Federal Circuit affirmed-in-part, vacated-in-part, and remanded-in-part, the PTAB’s determination that claims 1-8 of U.S. Patent No. 6,319,006 were unpatentable, while claims 9-10 were patentable.
The patent related to a drill template, to precisely place a pilot hole for a tooth implant. The parties each provided their own translation of the primary references, and based upon these different translations disputed whether the reference digitally inputs structures of the mouth or movements of the mouth into the simulation. Sirona focused on the references disclosure of a “recording bow,” which only measures movement of the jaw joint, not surface structures. However, the Federal Circuit agreed with the district court that this was not dispositive in light of the reference’s other disclosures.
In addition to agreeing with the Board about the content of the prior art, the Federal Circuit also found substantial evidence also supported the Board’s finding that a person of ordinary skill in the art would have been motivated to combine the references, and affirmed the determination that claims 1-8 would have been obvious.
Sirona also complained that the Board violated the APA when it determined that Sirona’s recording bow argument was not relevant and put together its own obviousness theory based on Bannuscher’s input of “geometry data,” which
does not appear in the petition. While the Federal Circuit agreed that it would not be proper for the Board to deviate from the grounds in the petition and raise its own obviousness theory, it held that the Board’s unpatentability determination did not
deviate from the grounds alleged in the petition. The Federal Circuit noted that the Board cited the same portions of the reference as the Petition, and said the Board “did not change theories simply because the petition did not use the exact words.” The Federal Circuit also noted that Sirona spent much of its response addressing the very argument that it complained was new.
Sirona also challenged the denial of its contingent motion to amend. On this point, the Federal Circuit agreed. The final written decision, which issued prior to Aqua Products,
improperly placed the burden on Sirona to demonstrate that the proposed substitute claims were patentable. The Federal Circuit vacated the Board’s denial of Sirona’s contingent motion to amend and remand for the Board to reconsider in light of Aqua Products.
On the cross appeal, the Federal Circuit affirmed the finding that claims 9 and 10 were patentable, noting that there was no error in the Board’s decision not to decide grounds of unpatentability not raised in the petition.
June 14, 2018, is Flag Day
Americans are proud of our flag, and the records of the U.S. Patent and Trademark Office, document a wide array of creative ways we Americans have found to display the flag, including on decorative displays (U.S. Patent No. 89,270):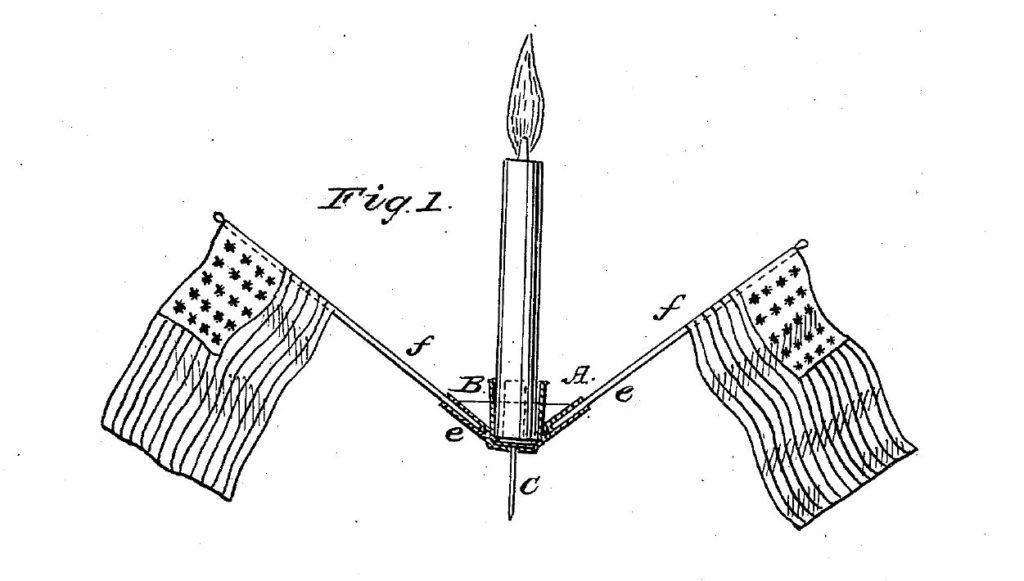 on parking meters (U.S. Patent No. 2,686,029):
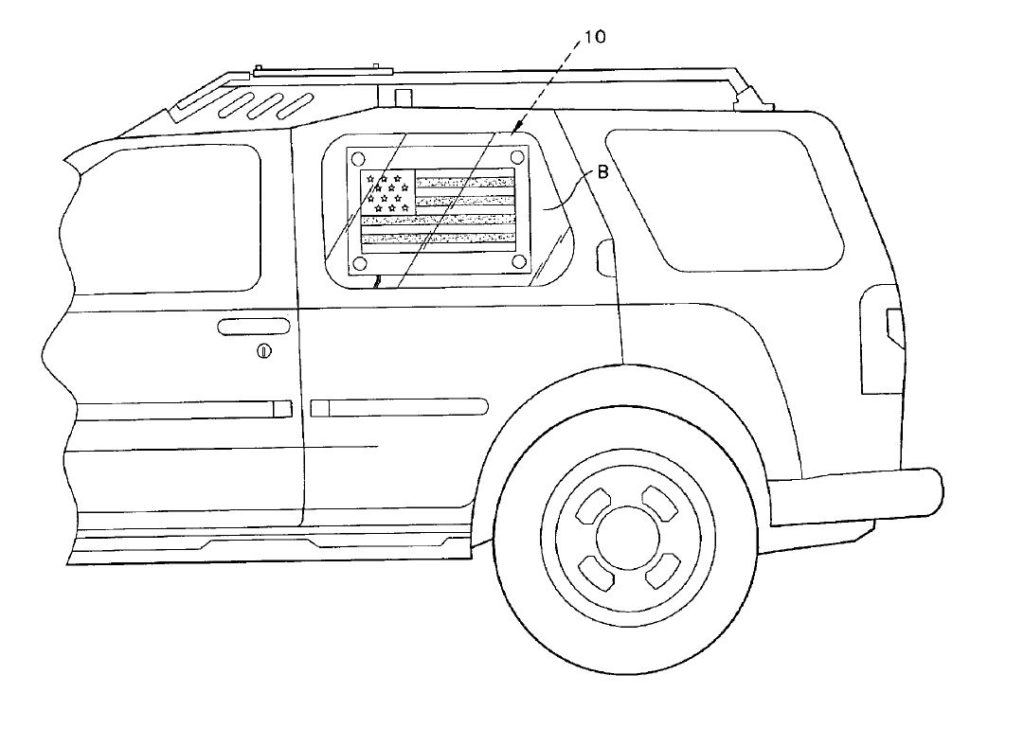
on parking meters (U.S. Patent No. 2,686,029):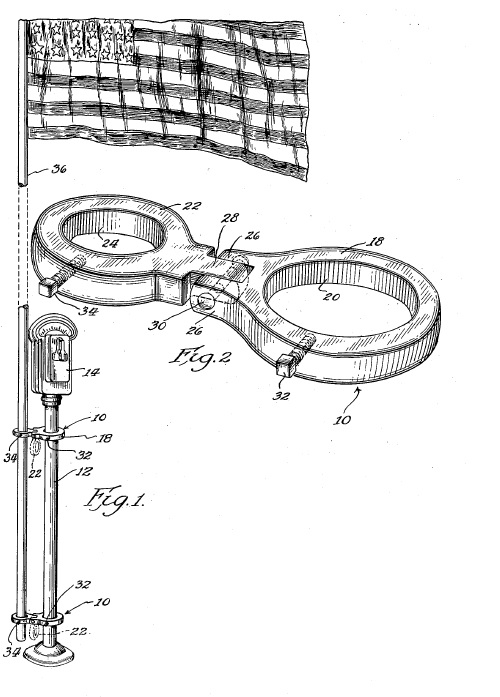 on our SUV’s (U.S. Patent No. 6,860,047):
on our SUV’s (U.S. Patent No. 6,860,047):
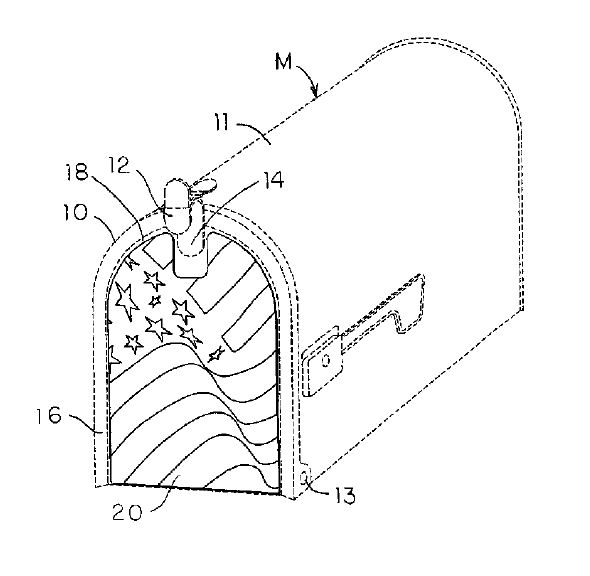
on our mailboxes (U.S. Patent No. 6,929,173):
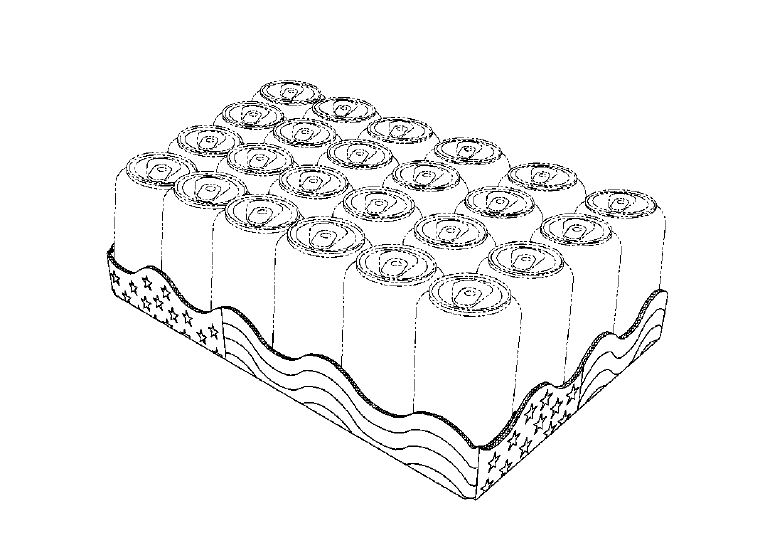
on our beverages (U.S. Patent No. D695626):
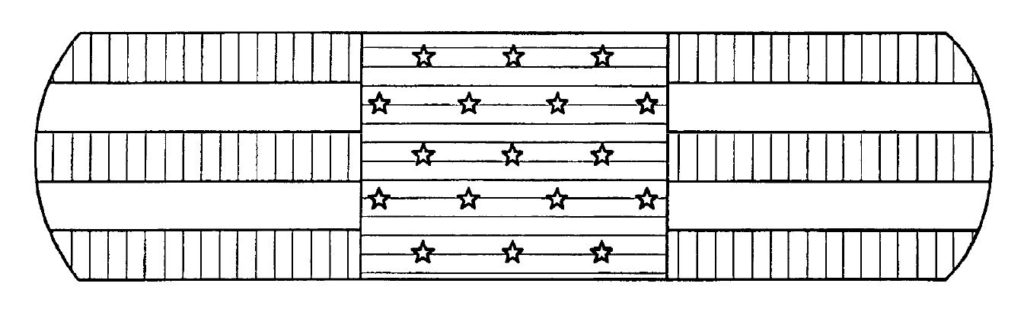
and even on our bandages (U.S. Patent No. D516730):
Salute the flag, where ever you find it!