In Campbell Soup Company v. Gamon Plus, Inc., [2020-2344, 2021-1019](August 19, 2021), the Federal Circuit reversed the PTAB determination that U.S. Design Patent Nos. D612,646 and D621,645 would not have been obvious.
The ’646 and ’645 patents, which each claim “[t]he ornamental design for a gravity
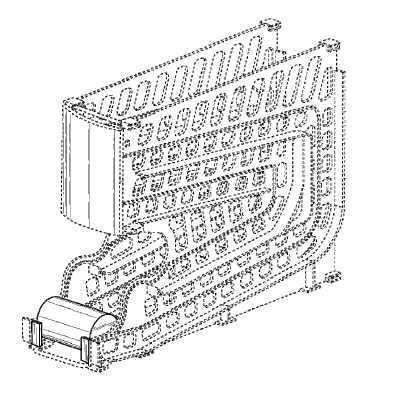
feed dispenser display, as shown and described. The ‘646 patent has one figure:
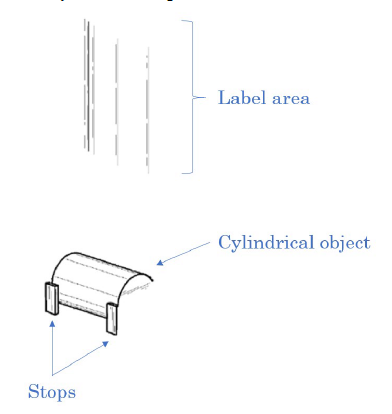
Most of the features in the drawings are shown in dashed lines meaning that they are not part of the claimed design. In the ‘646 patent, the only parts claimed, were athe label area, the can stops, and one of the cans:
The ‘645 patent similarly excludes most of the drawing, except the label area and the cylindrical object in the display.
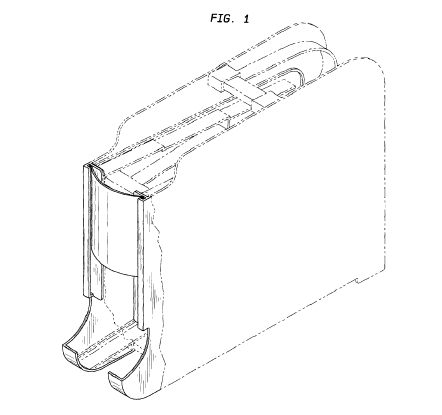
Linz, discloses a similar dislay rack. that has a similar display area and stops:
After buying $31 million of display racks from Gamon, Campbell’s began buying similar displays from Trinity, which caused Gamon to sue Campbells. Campbells petitioned for inter partes review of Gamon’s patents. The Board held that Appellants had failed to prove unpatentability, finding that Linz is not similar
enough to the claimed designs to constitute a proper primary reference.
The Federal Circuit vacated and remanded, reasoning that the “ever-so-slight differences” the Board identified between Linz and the claimed designs did not
support its finding that Linz is not a proper primary reference. On remand, the Board again help that the patents had not been shown to be obvious. The Board reasoned Abbate was not a proper primary reference, and while Linz was a proper primary references, the claimed designs would not have been obvious over Linz alone or in combination with other references. The Board acknowledged that Linz had the same overall visual appearance as the claimed designs, but objective indicia nonobviousness, namely: commercial success, Campbell praise for the design, and the copying of the design.
On this second trip to the Federal Circuit the Federal Circuit agreed that Linz creatd the same overall visual appearance as the claimed designs. However, as to objective indicia, the Federal Circuit found that the Board erred in presuming a nexus between any commercial success and the claimed designs. The Board found that the unclaimed portions of the desings were insignificant to the ornamental design, but the Federal Circuit held that: “[i]n determining coextensiveness, the question is not whether unclaimed features are insignificant to a product’s ornamental design. The question is instead whether unclaimed features are
“insignificant,” period. The Federal Circuit explained that the purpose of the coextensiveness requirement is to ensure that nexus is presumed only when the
product “is the invention disclosed and claimed. The Federal Circuit said by limiting its analysis to ornamental significance, the Board simply did not answer the relevant
question: whether the commercial produtc “is the invention.”
Under the correct legal standard, substantial evidence does not support the Board’s finding of coextensiveness, the Federal Circuit finding that at most, the claims cover only a small portion of the commercial product: its label area, cylindrical object, and stops. Thus it was improper to presume a nexus between the claimed invention and the commercial success, and it was up to Gamon to establish a nexus, which it failed to do.
The Board found nexus from the success and praise for the label area, but the Federal Circuit pointed out, this was not new. To establish nexus, Gamon needed to present evidence that the commercial success and praise of the commercial product derived from those “unique characteristics.” The evidence of commerical success presented was tied only to the label area, which was old. The Federal Circuit rejected testimony of the inventor tying the commercial success to the label area, noting that it was both self-serving and unssupported by any other evidence. Furthermore the Federal Circuit rejected the Boards view that in design patent cases, objective indicia need not be linked to the claimed design’s unique characteristics.
The Federal Circuit also relied upon evidence of copying as establishing non-obviousness, saying that even if there were copying, that alone did not overcome the strong evidence if obviousness.
Weighing all of the Graham factors, including (1) the Board’s finding that, from the perspective of a designer of ordinary skill, Linz creates the same overall visual impression as the claimed designs and (2) copying by Trinity of the claimed designs’ unique characteristics, the Federal Circuit concluded that the claimed designs would have been obvious over Linz.