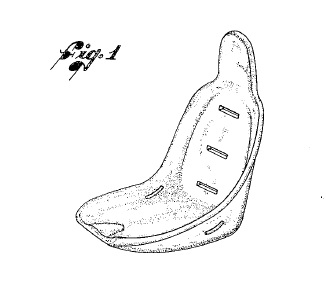
On February 2, 1971, actor Steve T. McQueen was issued U.S. Patent No. D219813 on a Bucket Seat Shell:
February 1 Patent of the Day
January 31 Patent of the Day
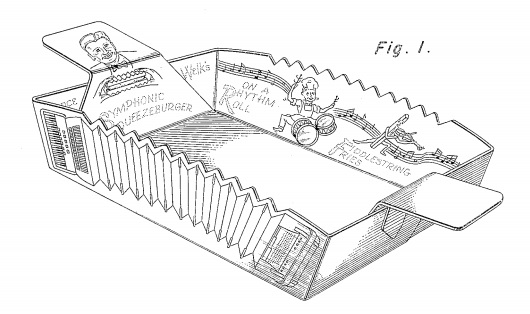
On January 31, 1950, Lawrence Welk (ask your grandparents) received U.S. Patent No. D157,110, on a Lunch Box. Check out the accordion pleats:
A “Symphony Burger” is still available at the Canyon Grille at the Welk Resort in Escondido, California, but there is no word on what happened to the Fiddlestring Fries.
January 30 Patent of the Day
January 29 Patent of the Day
January 28 Patent of the Day
January 27 Patent of the Day
January 26 Patent of the Day
On January 26, 1965, Noah McVicker and Joseph McVicker received U.S. Patent No. 3,167,440 on Plastic Modeling Composition of a Soft, Pliable Working Consistency, know to millions of children as Play-Doh. The product was based upon a wall paper cleaner that had be repurposed as a modeling compound.
Billions of cans of Play-Doh have been sold, and it can be found in more than 6,000 stores in the U.S., and in at least 75 countries around the world.
January 25 Patent of the Day
January 24 Patent of the Day
On January 24, 1922, U.S. Patent No. 1,404,539 issued to Christian K. Nelson on a “Confection” that was marketed as the Eskimo Pie.