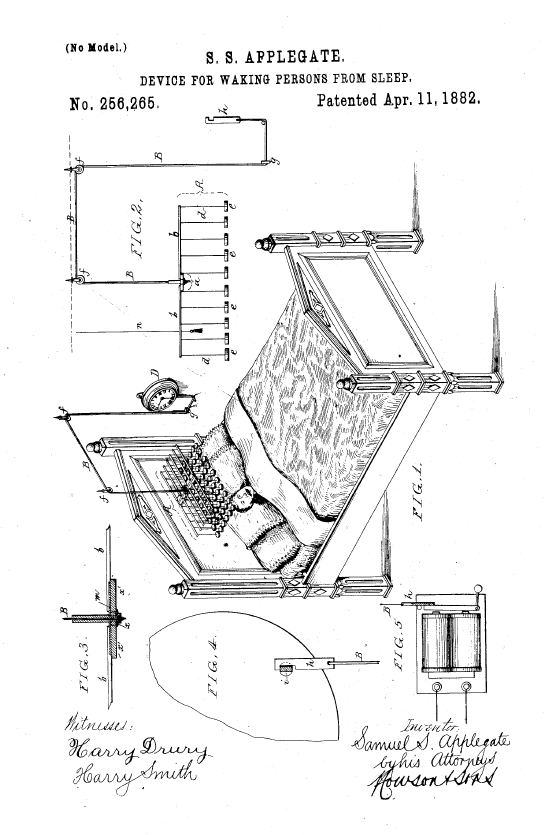
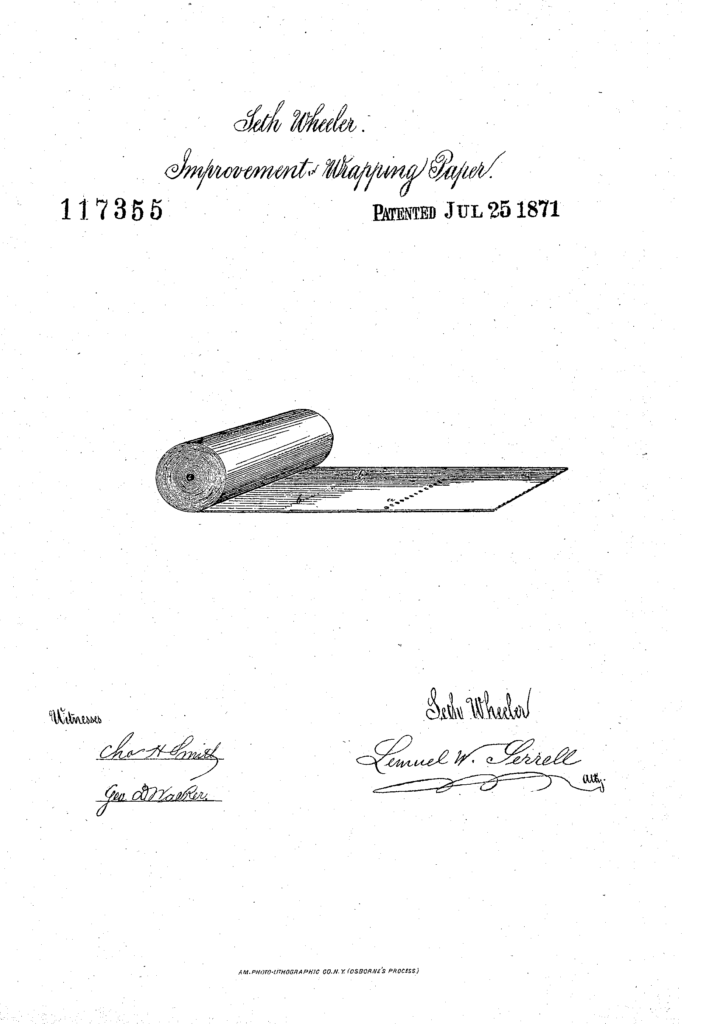
On July 25, 1871, Seth Wheeler received U.S. Patent No. 117,355, on Improvement in Wrapping-Papers — the improvement being providing the paper in a perforated roll:
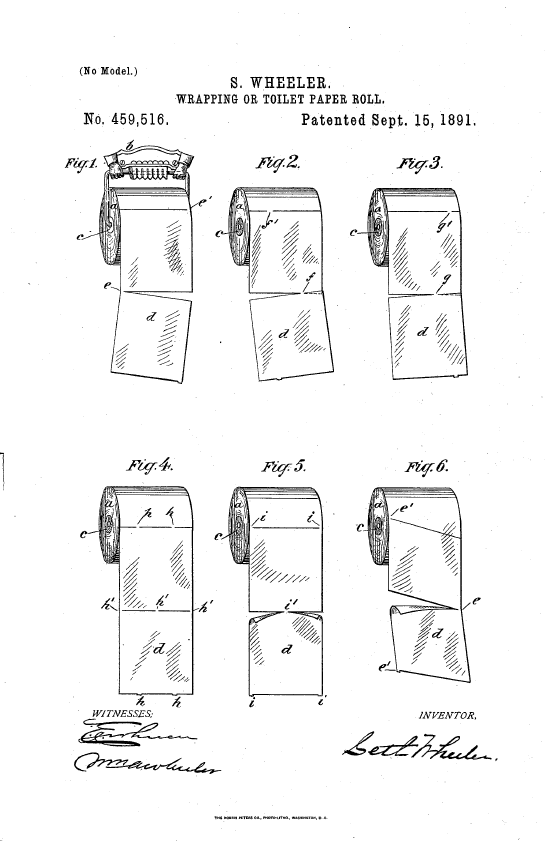
As Wheeler explained in his patent, previously wrapping paper was sold in individual sheets, which were difficult (and expensive) to deal with. The concept of perforations must have been percolating in his head because twenty years later, he invented and patented (U.S. Patent No. 459,516) a roll of perforated toilet paper:
Wheeler followed up just a few months later with U.S. Patent No. 465,588 on a Toilet-Paper Roll, having improved perforations for easy tearing,
The significance of Wheelers patents cannot be overstated, for they settle once and for all that the paper should hang from the front of the roll (not the back of the roll). If Wheeler were alive today he no doubt would flush with pride over how essential his invention became to modern life.