Novartis v. Lee and Exelixis v. Lee (2013-1160, 2013-1179, 2013-1175, Fed. Cir. 2014)
Summary: For a patent application issuing after 3 years, an RCE filed at any time during prosecution will reduce patent term adjustment (PTA) of the patent (i.e., the component of PTA referred to as “B Delay”). The amount of reduction in PTA is the period from the day the RCE was filed to the day a Notice of Allowance was sent (not to the day the patent issued).
Practice Note: It may be necessary to request PTA adjustments for recently issued patents and allowed applications that benefit from these decisions, as the USPTO will not automatically increase PTA of a patent that has already issued. At the latest, requests for reconsideration of PTA must be filed with the USPTO within 2 months of patent issuance.[i] Civil action challenges to PTA must be filed within 180 days from patent issuance.[ii] Unless successfully appealed to the Supreme Court, these decisions remove the basis that many Patentees had recently used for requesting additional PTA based on the lower courts’ decisions. For previous timely filed requests for reconsideration of PTA (challenging the PTO calculations based on the district court Exelixis and Novartis decisions), the USPTO held these in abeyance awaiting the Federal Circuit decisions. As of January 20, 2014, the USPTO had not yet issued guidelines on how these pending requests will be handled in view of the January 15, 2014 Federal Circuit decisions.
Discussion: The terms of many recently issued and future patents will be impacted by the companion Federal Circuit decisions in Novartis and Exelixis. Patent term adjustment (PTA) compensates a Patentee for certain delays caused by the USPTO during patent prosecution (by adding more term to a patent according to 35 U.S.C. 154).[iii] However, the USPTO delays may be offset by certain delays attributed to the Patentee. The USPTO determines PTA for each patent it issues based on its interpretation of the statute (35 U.S.C. 154(b)). Unfortunately, the statute is ambiguous and the USPTO’s interpretations have been subject to various challenges, including in the companion Exelixis and Novartis cases.
In each of Exelixis and Novartis, the Patentee disputed the USPTO’s calculation of PTA. The USPTO found that the Patentee’s filing of a request for continued examination (RCE) negated certain delays caused by the USPTO. Those familiar with prosecution before the USPTO are aware that RCEs are filed regularly. Thus, these companion decisions will likely impact the adjustment of terms of many patents in the future.
The statute divides the USPTO’s potential delays into “A Delay,” “B Delay,” and “C Delay.” B Delay was at issue here, which is a “[g]uarantee of no more than 3-year application pendency.” 35 U.S.C. 154(b)(1)(B).[iv] If the USPTO fails to issue a patent within 3 years after the application filing date, the patent term will be extended by one day for each day beyond the 3-year deadline. However, the statute also provides that certain Patentee delays offset the USPTO delay. These Patentee delays include, among others, “any time consumed by continued examination of the application requested by applicant.”
The Exelixis and Novartis decisions depend on statutory interpretation of the language “not including — any time consumed by continued examination.” It was undisputed that filing an RCE before the USPTO’s 3 year deadline would reduce the PTO B Delay after the 3-year deadline. However, whether filing an RCE after the 3-year period had passed would also reduce the USPTO B Delay (who already had not met the 3-year deadline) was at issue. If the USPTO’s interpretation was incorrect as the lower courts held, the filing of an RCE after the 3-year deadline did not count towards reducing the USPTO B Delay. Many patents, including those here, would have significant amounts of term added. However, the Federal Circuit endorsed the USPTO’s interpretation that the filing of an RCE by an applicant at any time during prosecution (regardless of whether before or after the 3 year mark) is considered to be Patentee delay that will offset the USPTO B Delay (for not meeting the 3 year deadline).
Another issue was how much time should be attributed to Patentee’s RCE delay. Did the delay extend from filing the RCE to (1) notice of allowance or (2) actual issuance (as the USPTO calculated)? The Federal Circuit sided with the Patentees finding the USPTO was incorrectly calculating the amount of this delay. The delay attributable to a Patentee for filing an RCE only extends until the notice of allowance is sent, not until issuance of the patent. Thus, in the future, the USPTO will now have to adjust its PTA calculations to include its delays between the notice of allowance and issuance for PTA (assuming that this time is past the 3-year deadline). Presumably, this holding also applies to any 35 U.S.C. 154(b)(1)(i)-(iii) delays, including filing of appeals and appellate review. Accordingly, promulgation of new regulations (37 C.F.R. 1.703(b)) by the USPTO should also be forthcoming.
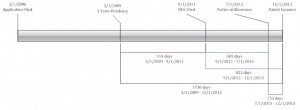
Example: To illustrate how this case will apply to PTA calculation, a simplified example is below (focusing on B Delays).
The application was filed on 3/1/2006.
The 3-year pendency period ended on 3/1/2009.
The RCE was filed on 9/1/2011.
A notice of allowance was issued on 7/1/2013.
Pre-Exelixis and Novartis, the USPTO would likely have calculated PTA to be 914 days. The B Delay is 1,736 days (from the 3-year date to issuance). This would be offset by Patentee delay due to the RCE of 822 days (from the filing of the RCE to issuance). PTA is USPTO Delay (including the B Delay of 1,736 days + A Delay + C Delay) – (Patentee Delays of 822 days + Overlapping Days between (A and B Delays) or (A and C Delays)). Because A and C delays are neglected here for simplicity, PTA based on B Delay would be 914 days.
Now, however, the USPTO delay of 1,736 days is only offset by 669 days (from the filing of the RCE to allowance). This results in a B delay of 1,067 days (an increase of 153 days over the old method).
Alternatively, for RCEs filed after the 3 year date, the B Delay can be seen as the sum of a first period of 914 days (from the 3 year date to the filing of the RCE) and a second period of 153 days (from the notice of allowance to issuance), for a total of 1,067 days.
[i] 37 C.F.R. 1.705.
[ii] 35 U.S.C. 154(b)(4)(A).
[iii] U.S. patent term is provided by 35 U.S.C. 154. 35 U.S.C. 154(a)(2) in particular provides:
Term.— Subject to the payment of fees under this title, such grant shall be for a term beginning on the date on which the patent issues and ending 20 years from the date on which the application for the patent was filed in the United States or, if the application contains a specific reference to an earlier filed application or applications under section 120, 121, or 365(c), from the date on which the earliest such application was filed.
[iv] 35 U.S.C. 154(b) Adjustment of Patent Term.—
(1) Patent term guarantees.—
…
(B) Guarantee of no more than 3 year application pendency.— Subject to the limitations under paragraph (2), if the issue of an original patent is delayed due to the failure of the United States Patent and Trademark Office to issue a patent within 3 years after the actual filing date of the application under section 111(a) in the United States or, in the case of an international application, the date of commencement of the national stage under section 371 in the international application, not including—
(i) any time consumed by continued examination of the application requested by the applicant under section 132(b);
(ii) any time consumed by a proceeding under section 135(a), any time consumed by the imposition of an order under section 181, or any time consumed by appellate review by the Patent Trial and Appeal Board or by a Federal court; or
(iii) any delay in the processing of the application by the United States Patent and Trademark Office requested by the applicant except as permitted by paragraph (3)(C), the term of the patent shall be extended 1 day for each day after the end of that 3 year period until the patent is issued.
Emphases added.